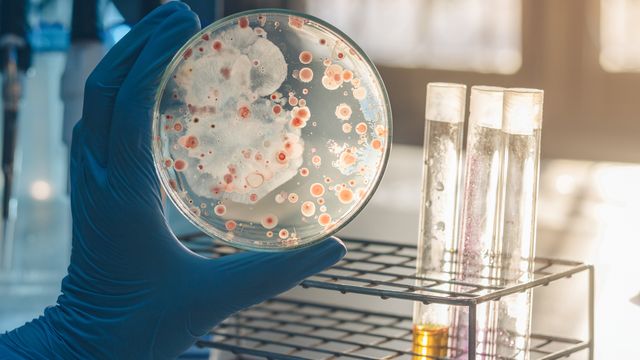
Vibrio parahaemolyticus Culture Testing in Shellfish
Shellfish are a significant component of global seafood consumption. However, they can also serve as vectors for various pathogens, including Vibrio parahaemolyticus. This bacterium is a major cause of foodborne illness worldwide and can lead to severe gastroenteritis if consumed in contaminated shellfish.
Microbiological testing aimed at detecting and quantifying V. parahaemolyticus in shellfish is critical for ensuring public health and maintaining consumer confidence in seafood products. The presence of this pathogen can be particularly insidious, as it may not cause visible spoilage or discoloration but still pose a significant risk to human health.
The Culture Media used for the isolation and enumeration of V. parahaemolyticus in shellfish must meet stringent criteria outlined by international standards such as ISO 16867-2, which specifies methods for the detection of this pathogen in aquatic products.
The testing process involves several critical steps:
- Sample Collection: Shellfish are collected from various sources and transported to our laboratory under controlled conditions.
- Preparation: Samples undergo thorough preparation, including homogenization and dilution, to ensure accurate microbial counts.
- Culture Incubation: Prepared samples are inoculated onto specialized media suitable for V. parahaemolyticus, which allows the pathogen to grow under specific conditions.
- Identification: Once colonies form, they undergo further identification using biochemical tests and possibly PCR methods.
- Quantification: The number of V. parahaemolyticus is quantified based on colony-forming units (CFUs).
- Reporting: Results are compiled into comprehensive reports, which include statistical analysis and compliance with regulatory limits.
The importance of this testing cannot be overstated. Not only does it safeguard public health but also helps in maintaining the integrity of the seafood industry's reputation. Regulatory bodies such as the U.S. FDA and EU impose strict limits on the allowable levels of V. parahaemolyticus to ensure food safety.
The accurate identification and quantification of this pathogen can also guide quality assurance practices in shellfish cultivation, processing, and distribution. For instance, if a high presence is detected, corrective actions such as increased hygiene standards or changes in harvesting protocols may be necessary.
Why It Matters
The significance of Vibrio parahaemolyticus culture testing extends beyond the laboratory. Ensuring food safety is a shared responsibility, and shellfish producers, processors, and distributors play critical roles in preventing contamination.
Health Risks: Ingestion of contaminated shellfish can lead to symptoms such as diarrhea, nausea, vomiting, abdominal cramps, and fever. Severe cases may require hospitalization. The World Health Organization (WHO) reports that Vibrio infections are among the most common causes of foodborne illness globally.
Regulatory Compliance: Failure to meet regulatory standards can result in product recalls, financial losses, and reputational damage. Regulatory bodies like the FDA impose strict limits on the allowable levels of V. parahaemolyticus. For example, according to FDA regulations, the count should not exceed 235 CFU/g for raw shellfish.
Consumer Confidence: Maintaining high standards in food safety fosters trust among consumers and supports market growth. A single incident of contamination can significantly impact consumer behavior and industry profitability.
Economic Impact: The economic implications are substantial, affecting not only the seafood industry but also related sectors such as tourism. Contaminated shellfish can lead to a ban on exports or restrictions on certain species, causing financial losses for producers and processors.
Why Choose This Test
The choice of Vibrio parahaemolyticus culture testing in shellfish is driven by several key factors:
Reliable Results: Our laboratory employs state-of-the-art equipment and experienced microbiologists to ensure accurate and reliable results. We use internationally recognized methods, such as ISO 16867-2, to guarantee compliance with global standards.
Comprehensive Reporting: Besides quantification, we provide detailed reports that include statistical analysis, trends over time, and comparisons with regulatory limits. This information is invaluable for stakeholders looking to make informed decisions.
Expertise and Experience: Our team of microbiologists has extensive experience in food safety testing, including shellfish microbiology. Their expertise ensures that we stay at the forefront of industry practices and standards.
Client Support: We offer personalized support to help clients understand their results and implement necessary corrective actions. Our goal is not just to test but also to ensure ongoing compliance with food safety regulations.
Cost-Effective Solutions: While ensuring the highest standards of quality, our services are designed to be cost-effective, minimizing operational costs for our clients without compromising on accuracy or reliability.
International Acceptance and Recognition
- Australia: The National Association of Testing Authorities (NATA) recognizes our testing methods for shellfish microbiology.
- Canada: Our laboratory adheres to the guidelines set by Health Canada, ensuring that test results are accepted across the country.
- Europe: We comply with EU standards and are recognized by the European Food Safety Authority (EFSA).
- Japan: Our methods meet the stringent requirements of the Japanese Ministry of Health, Labour and Welfare.
- New Zealand: The New Zealand Food Safety Science & Technology Centre accredits our testing for shellfish safety.
- Singapore: Our laboratory is recognized by the Singapore National Environment Agency (NEA).
- United Kingdom: We are accredited by the United Kingdom Accreditation Service (UKAS) to ISO/IEC 17025 for microbiological testing.
- United States: Our methods comply with FDA requirements and are recognized by the U.S. Department of Agriculture (USDA).